What Are Two Ways Nitrogen Is Changed to a Form Plants Can Use
Nitrogen is one of the primary nutrients critical for the survival of all living organisms. Although nitrogen is very abundant in the atmosphere, information technology is largely inaccessible in this form to most organisms. This article explores how nitrogen becomes available to organisms and what changes in nitrogen levels as a issue of human action ways to local and global ecosystems.
Introduction
Nitrogen is i of the main nutrients critical for the survival of all living organisms. It is a necessary component of many biomolecules, including proteins, Dna, and chlorophyll. Although nitrogen is very abundant in the atmosphere as dinitrogen gas (Ntwo), it is largely inaccessible in this form to virtually organisms, making nitrogen a scarce resource and often limiting primary productivity in many ecosystems. Only when nitrogen is converted from dinitrogen gas into ammonia (NHthree) does it become available to principal producers, such as plants.
In improver to Ntwo and NHthree, nitrogen exists in many unlike forms, including both inorganic (east.g., ammonia, nitrate) and organic (e.g., amino and nucleic acids) forms. Thus, nitrogen undergoes many different transformations in the ecosystem, changing from one grade to another as organisms apply information technology for growth and, in some cases, energy. The major transformations of nitrogen are nitrogen fixation, nitrification, denitrification, anammox, and ammonification (Figure ane). The transformation of nitrogen into its many oxidation states is key to productivity in the biosphere and is highly dependent on the activities of a various aggregation of microorganisms, such every bit bacteria, archaea, and fungi.
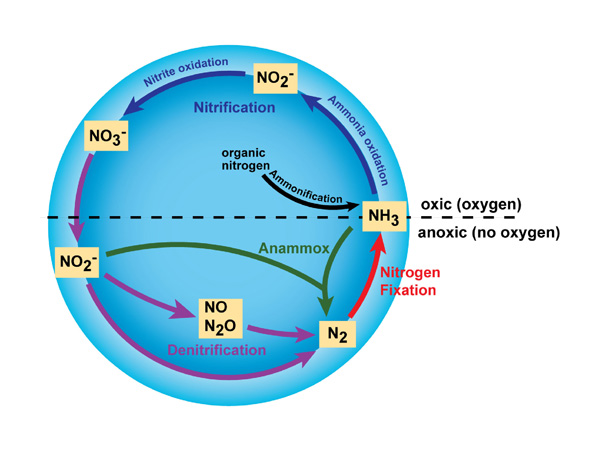
Figure one: Major transformations in the nitrogen bicycle
Since the mid-1900s, humans have been exerting an ever-increasing touch on on the global nitrogen wheel. Homo activities, such as making fertilizers and burning fossil fuels, take significantly altered the amount of fixed nitrogen in the Earth's ecosystems. In fact, some predict that by 2030, the amount of nitrogen fixed by human activities will exceed that fixed by microbial processes (Vitousek 1997). Increases in available nitrogen can alter ecosystems by increasing primary productivity and impacting carbon storage (Galloway et al. 1994). Considering of the importance of nitrogen in all ecosystems and the significant impact from human activities, nitrogen and its transformations have received a great deal of attention from ecologists.
Nitrogen Fixation
Nitrogen gas (North2) makes up virtually 80% of the Earth'due south temper, yet nitrogen is often the nutrient that limits chief production in many ecosystems. Why is this so? Because plants and animals are not able to use nitrogen gas in that grade. For nitrogen to be available to make proteins, Dna, and other biologically important compounds, it must get-go exist converted into a different chemical grade. The process of converting N2 into biologically available nitrogen is called nitrogen fixation. Due north2 gas is a very stable chemical compound due to the strength of the triple bond between the nitrogen atoms, and information technology requires a large amount of free energy to break this bail. The whole process requires eight electrons and at least xvi ATP molecules (Figure 2). As a result, just a select group of prokaryotes are able to bear out this energetically demanding process. Although about nitrogen fixation is carried out by prokaryotes, some nitrogen can be stock-still abiotically by lightning or certain industrial processes, including the combustion of fossil fuels.
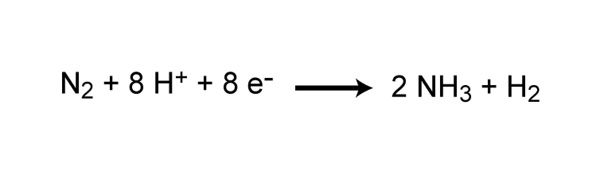
Figure two: Chemical reaction of nitrogen fixation

Effigy 3: Nitrogen-fixing nodules on a clover plant root
Some nitrogen-fixing organisms are free-living while others are symbiotic nitrogen-fixers, which require a close association with a host to carry out the procedure. Near of the symbiotic associations are very specific and have complex mechanisms that assistance to maintain the symbiosis. For example, root exudates from legume plants (e.g., peas, clover, soybeans) serve as a bespeak to certain species of Rhizobium, which are nitrogen-fixing bacteria. This signal attracts the bacteria to the roots, and a very complex series of events then occurs to initiate uptake of the bacteria into the root and trigger the process of nitrogen fixation in nodules that form on the roots (Figure iii).
Some of these bacteria are aerobic, others are anaerobic; some are phototrophic, others are chemotrophic (i.eastward., they use chemicals equally their free energy source instead of lite) (Tabular array one). Although there is great physiological and phylogenetic diverseness amongst the organisms that acquit out nitrogen fixation, they all take a similar enzyme complex called nitrogenase that catalyzes the reduction of N2 to NHthree (ammonia), which can be used as a genetic marker to identify the potential for nitrogen fixation. I of the characteristics of nitrogenase is that the enzyme complex is very sensitive to oxygen and is deactivated in its presence. This presents an interesting dilemma for aerobic nitrogen-fixers and particularly for aerobic nitrogen-fixers that are also photosynthetic since they really produce oxygen. Over fourth dimension, nitrogen-fixers have evolved different ways to protect their nitrogenase from oxygen. For example, some cyanobacteria accept structures called heterocysts that provide a depression-oxygen environment for the enzyme and serves as the site where all the nitrogen fixation occurs in these organisms. Other photosynthetic nitrogen-fixers fix nitrogen just at night when their photosystems are fallow and are not producing oxygen.
Genes for nitrogenase are globally distributed and take been found in many aerobic habitats (e.one thousand., oceans, lakes, soils) and also in habitats that may be anaerobic or microaerophilic (eastward.thousand., termite guts, sediments, hypersaline lakes, microbial mats, planktonic crustaceans) (Zehr et al. 2003). The broad distribution of nitrogen-fixing genes suggests that nitrogen-fixing organisms display a very broad range of environmental weather condition, as might be expected for a process that is disquisitional to the survival of all life on World.
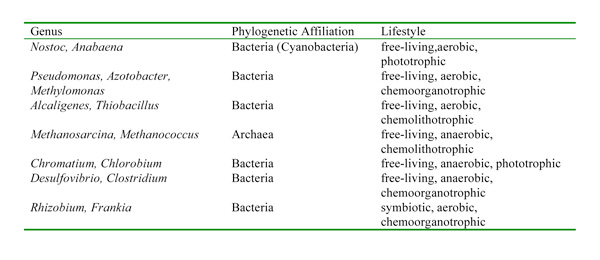
Table one: Representative prokaryotes known to comport out nitrogen fixation
Nitrification
Nitrification is the process that converts ammonia to nitrite and and so to nitrate and is some other important step in the global nitrogen cycle. Most nitrification occurs aerobically and is carried out exclusively by prokaryotes. In that location are ii singled-out steps of nitrification that are carried out by singled-out types of microorganisms. The start footstep is the oxidation of ammonia to nitrite, which is carried out by microbes known as ammonia-oxidizers. Aerobic ammonia oxidizers convert ammonia to nitrite via the intermediate hydroxylamine, a process that requires two dissimilar enzymes, ammonia monooxygenase and hydroxylamine oxidoreductase (Figure 4). The process generates a very small amount of energy relative to many other types of metabolism; equally a result, nitrosofiers are notoriously very ho-hum growers. Additionally, aerobic ammonia oxidizers are also autotrophs, fixing carbon dioxide to produce organic carbon, much like photosynthetic organisms, but using ammonia as the energy source instead of low-cal.
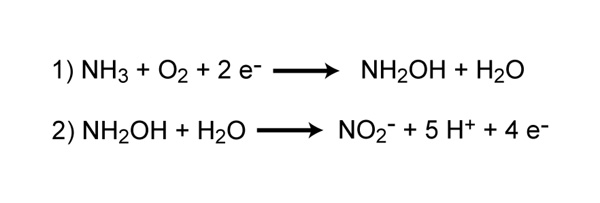
Figure four: Chemical reactions of ammonia oxidation carried out by leaner
Reaction ane converts ammonia to the intermediate, hydroxylamine, and is catalyzed by the enzyme ammonia monooxygenase. Reaction 2 converts hydroxylamine to nitrite and is catalyzed by the enyzmer hydroxylamine oxidoreductase.
Dissimilar nitrogen fixation that is carried out by many different kinds of microbes, ammonia oxidation is less broadly distributed amid prokaryotes. Until recently, it was thought that all ammonia oxidation was carried out past only a few types of bacteria in the genera Nitrosomonas, Nitrosospira, and Nitrosococcus. However, in 2005 an archaeon was discovered that could also oxidize ammonia (Koenneke et al. 2005). Since their discovery, ammonia-oxidizing Archaea have often been found to outnumber the ammonia-oxidizing Bacteria in many habitats. In the past several years, ammonia-oxidizing Archaea have been institute to be arable in oceans, soils, and table salt marshes, suggesting an important role in the nitrogen cycle for these newly-discovered organisms. Currently, merely one ammonia-oxidizing archaeon has been grown in pure civilization, Nitrosopumilus maritimus, so our agreement of their physiological variety is express.
The 2nd step in nitrification is the oxidation of nitrite (NOtwo -) to nitrate (NO3 -) (Figure 5). This step is carried out by a completely separate grouping of prokaryotes, known as nitrite-oxidizing Leaner. Some of the genera involved in nitrite oxidation include Nitrospira, Nitrobacter, Nitrococcus, and Nitrospina. Like to ammonia oxidizers, the energy generated from the oxidation of nitrite to nitrate is very minor, and thus growth yields are very low. In fact, ammonia- and nitrite-oxidizers must oxidize many molecules of ammonia or nitrite in club to fix a single molecule of COii. For complete nitrification, both ammonia oxidation and nitrite oxidation must occur.
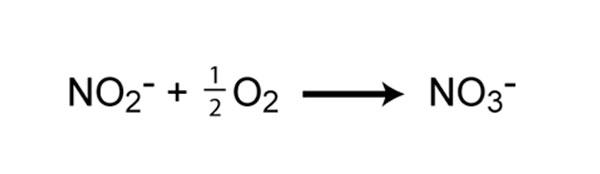
Figure 5: Chemical reaction of nitrite oxidation
Ammonia-oxidizers and nitrite-oxidizers are ubiquitous in aerobic environments. They accept been extensively studied in natural environments such as soils, estuaries, lakes, and open-ocean environments. However, ammonia- and nitrite-oxidizers also play a very important role in wastewater treatment facilities by removing potentially harmful levels of ammonium that could lead to the pollution of the receiving waters. Much inquiry has focused on how to maintain stable populations of these important microbes in wastewater handling plants. Additionally, ammonia- and nitrite-oxidizers help to maintain healthy aquaria by facilitating the removal of potentially toxic ammonium excreted in fish urine.
Anammox
Traditionally, all nitrification was thought to exist carried out nether aerobic weather condition, but recently a new blazon of ammonia oxidation occurring under anoxic atmospheric condition was discovered (Strous et al. 1999). Anammox (anaerobic ammonia oxidation) is carried out by prokaryotes belonging to the Planctomycetes phylum of Leaner. The first described anammox bacterium was Brocadia anammoxidans. Anammox bacteria oxidize ammonia by using nitrite every bit the electron acceptor to produce gaseous nitrogen (Effigy 6). Anammox bacteria were starting time discovered in anoxic bioreactors of wasterwater treatment plants just accept since been found in a variety of aquatic systems, including low-oxygen zones of the ocean, littoral and estuarine sediments, mangroves, and freshwater lakes. In some areas of the ocean, the anammox process is considered to be responsible for a significant loss of nitrogen (Kuypers et al. 2005). Withal, Ward et al. (2009) argue that denitrification rather than anammox is responsible for well-nigh nitrogen loss in other areas. Whether anammox or denitrification is responsible for most nitrogen loss in the sea, it is clear that anammox represents an important process in the global nitrogen cycle.
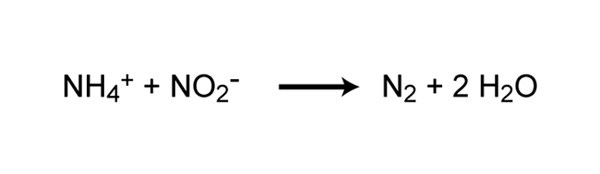
Figure 6: Chemical reaction of anaerobic ammonia oxidation (anammox)
Denitrification
Denitrification is the process that converts nitrate to nitrogen gas, thus removing bioavailable nitrogen and returning it to the atmosphere. Dinitrogen gas (N2) is the ultimate end product of denitrification, but other intermediate gaseous forms of nitrogen exist (Figure seven). Some of these gases, such as nitrous oxide (N2O), are considered greenhouse gasses, reacting with ozone and contributing to air pollution.
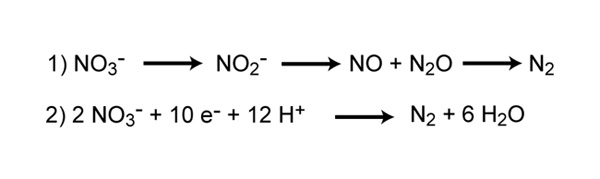
Effigy vii: Reactions involved in denitrification
Reaction one represents the steps of reducing nitrate to dinitrogen gas. Reaction 2 represents the consummate redox reaction of denitrification.
Unlike nitrification, denitrification is an anaerobic process, occurring mostly in soils and sediments and anoxic zones in lakes and oceans. Like to nitrogen fixation, denitrification is carried out by a diverse group of prokaryotes, and there is recent evidence that some eukaryotes are besides capable of denitrification (Risgaard-Petersen et al. 2006). Some denitrifying bacteria include species in the genera Bacillus, Paracoccus, and Pseudomonas. Denitrifiers are chemoorganotrophs and thus must also be supplied with some class of organic carbon.
Denitrification is important in that information technology removes fixed nitrogen (i.e., nitrate) from the ecosystem and returns it to the atmosphere in a biologically inert form (Northward2). This is particularly important in agriculture where the loss of nitrates in fertilizer is detrimental and plush. However, denitrification in wastewater handling plays a very benign role by removing unwanted nitrates from the wastewater effluent, thereby reducing the chances that the water discharged from the treatment plants will cause undesirable consequences (e.g., algal blooms).
Ammonification
When an organism excretes waste product or dies, the nitrogen in its tissues is in the course of organic nitrogen (e.g. amino acids, DNA). Various fungi and prokaryotes so decompose the tissue and release inorganic nitrogen back into the ecosystem every bit ammonia in the procedure known as ammonification. The ammonia then becomes available for uptake by plants and other microorganisms for growth.
Ecological Implications of Man Alterations to the Nitrogen Wheel
Many man activities have a significant bear on on the nitrogen cycle. Burning fossil fuels, application of nitrogen-based fertilizers, and other activities tin can dramatically increase the corporeality of biologically bachelor nitrogen in an ecosystem. And because nitrogen availability often limits the master productivity of many ecosystems, large changes in the availability of nitrogen can lead to severe alterations of the nitrogen wheel in both aquatic and terrestrial ecosystems. Industrial nitrogen fixation has increased exponentially since the 1940s, and deed has doubled the corporeality of global nitrogen fixation (Vitousek et al. 1997).
In terrestrial ecosystems, the addition of nitrogen can lead to food imbalance in trees, changes in forest wellness, and declines in biodiversity. With increased nitrogen availability there is often a modify in carbon storage, thus impacting more processes than but the nitrogen cycle. In agricultural systems, fertilizers are used extensively to increase plant production, merely unused nitrogen, unremarkably in the form of nitrate, can leach out of the soil, enter streams and rivers, and ultimately brand its way into our drinking water. The process of making synthetic fertilizers for employ in agronomics by causing N2 to react with H2, known every bit the Haber-Bosch process, has increased significantly over the past several decades. In fact, today, nearly 80% of the nitrogen institute in homo tissues originated from the Haber-Bosch process (Howarth 2008).
Much of the nitrogen applied to agricultural and urban areas ultimately enters rivers and nearshore littoral systems. In nearshore marine systems, increases in nitrogen can often atomic number 82 to anoxia (no oxygen) or hypoxia (low oxygen), altered biodiversity, changes in food-web construction, and general habitat degradation. 1 common consequence of increased nitrogen is an increase in harmful algal blooms (Howarth 2008). Toxic blooms of certain types of dinoflagellates have been associated with loftier fish and shellfish mortality in some areas. Even without such economically catastrophic furnishings, the addition of nitrogen can lead to changes in biodiversity and species composition that may lead to changes in overall ecosystem function. Some have fifty-fifty suggested that alterations to the nitrogen wheel may lead to an increased risk of parasitic and infectious diseases among humans and wild animals (Johnson et al. 2010). Additionally, increases in nitrogen in aquatic systems can pb to increased acidification in freshwater ecosystems.
Summary
Nitrogen is arguably the near of import nutrient in regulating primary productivity and species diversity in both aquatic and terrestrial ecosystems (Vitousek et al. 2002). Microbially-driven processes such every bit nitrogen fixation, nitrification, and denitrification, plant the bulk of nitrogen transformations, and play a critical role in the fate of nitrogen in the Earth's ecosystems. However, as homo populations go along to increase, the consequences of human activities go along to threaten our resource and have already significantly altered the global nitrogen cycle.
References and Recommended Reading
Galloway, J. Due north. et al. Yr 2020: Consequences of population growth and development on deposition of oxidized nitrogen. Ambio 23, 120–123 (1994).
Howarth, R. W. Littoral nitrogen pollution: a review of sources and trends globally and regionally. Harmful Algae viii, 14–20. (2008).
Johnson, P. T. J. et al. Linking environmental nutrient enrichment and disease emergence in humans and wildlife. Ecological Applications 20, 16–29 (2010).
Koenneke, M. et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437, 543–546 (2005).
Kuypers, Yard. M. M. et al. Massive nitrogen loss from the Benguela upwelling organisation through anaerobic ammonium oxidation. Proceedings of the National Academy of Sciences of the The states of America 102, 6478–6483 (2005).
Risgaard-Petersen, N. et al. Evidence for complete denitrification in a benthic foraminifer. Nature 443, 93–96 (2006).
Strous, Yard. et al. Missing lithotroph identified every bit new planctomycete. Nature 400, 446–449 (1999).
Vitousek, P. M. et al. Human alteration of the global nitrogen cycle: sources and consequences. Ecological Applications 7, 737–750 (1997).
Vitousek, P. K. et al. Towards an ecological agreement of biological nitrogen fixation. Biogeochemistry 57, ane–45 (2002).
Ward, B. B. et al. Denitrification every bit the dominant nitrogen loss process in the Arabian Sea. Nature 460, 78–81 (2009).
Zehr, J. P. et al. Nitrogenase gene multifariousness and microbial community structure: a cantankerous-system comparing. Environmental Microbiology 5, 539–554 (2003).
Source: https://www.nature.com/scitable/knowledge/library/the-nitrogen-cycle-processes-players-and-human-15644632/#:~:text=Thus%2C%20nitrogen%20undergoes%20many%20different,and%20ammonification%20(Figure%201).
0 Response to "What Are Two Ways Nitrogen Is Changed to a Form Plants Can Use"
Post a Comment